Carbon Removal: Engineering Solutions to Capture and Sequester Biomass Carbon
A concept model for fully exploiting photosynthesis for carbon capture
The DOE recently solicited an RFI (DE-FOA-0003421) seeking information focused on leveraging the zero-energy carbon dioxide capture process provided by photosynthesis to develop novel, transformational technologies that improve the energy efficiency of the carbon dioxide removal (CDR) sector.
ARPA-E eXCHANGE: Funding Opportunities (energy.gov)
The following is my edited submission to the DOE on this topic.
Potential Research Area #2: Optimizing Biomass Processing and Storage
This response to the Carbon Harvesting RFI will inform the DOE that a universal and fundamentally energy-efficient CO2 capture technology is possible. With this universal CO2 capture technology, biomass combustion to produce electricity will allow the capture and sequestration of the produced CO2 and have an energy surplus sufficient to capture and sequester 1.5 additional tons of CO2 from the air. The ash produced by combustion produces mineral fertilizers that recycle nutrients into the environment.
When this universal, fundamentally energy-efficient CO2 capture technology is developed (probably by the Chinese Government), worldwide CO2 capture utilizing biomass will be dominated by Chinese companies that earn 2.5 credits per unit of biomass (1-ton CO2 -eq).
Biomass-centric companies earning only a single credit per unit of biomass will be significantly disadvantaged and fail to acquire feedstock. Eventually, they will wither and die.
This response is organized into three sections:
General Discussion
a. Exploiting Artifacts of Nature
b. Properly Managing Biomass Chemical Exergy
c. Carbon Credits
d. Energy Efficient CO2 Capture
A concept model showing biomass combustion and DAC working together to remove CO2 from the environment.
a. Hub and Spoke Model
b. Costs
c. Community Benefits
A description of the CO2 capture chemistry that makes universal and energy-efficient CO2 capture possible.
a. Exergy Management – Harness or Destroy
b. Partial Pressure Staging
General Discussion
Unexploited Artifacts of Nature
Nature provides a range of phenomena that our civilization can harness for remarkable achievements. One such example is the observation that some materials glow when passing an electrical current. Harnessing this natural effect led to the development of the light bulb and the electrification of our civilization.
Our society advances by utilizing these natural principles or 'Artifacts of Nature'. One significant unexploited Artifact of Nature is the “Structure and Function of Water”. Living organisms exploit this artifact to achieve phenomenal success.
Consider the laws of thermodynamics, which dictate that capturing CO2 from lower concentrations requires more energy. For instance, capturing CO2 from ambient air, which contains about 400 parts per million (ppm), is far more energy-intensive than from a gas stream with 150,000 ppm.
Photosynthesis is another Artifact of Nature currently underutilized for carbon removal. While widely recognized for its role in producing oxygen and biomass, photosynthesis also naturally concentrates CO2. It takes in 400 ppm CO2 from the air and, through the process, creates biomass. When this biomass is burned, a gas is produced with a much higher CO2 concentration (around 150,000 ppm). Photosynthesis concentrates CO2 by a factor of 400.
However, we aren't yet using this process effectively for CO2 capture because current technologies do not properly utilize the exergy of biomass. To truly exploit photosynthesis for carbon capture, we need technologies that manage the chemical and carbon exergy more effectively, allowing us to capture CO2 with significantly less energy than is currently possible.
Biomass Chemical Exergy
Biomass used for CDR has exergy. The fact that biomass has exergy is indisputable. Produced by photosynthesis, biomass is a carbon-neutral material that contains significant chemical energy. Wood is an excellent example of exergy-containing biomass, which can be burned to produce heat slowly or quickly.
Burned slowly, biomass in a campfire or fireplace produces heat that keeps us warm. Burned aggressively, biomass can power a steam-powered locomotive or steamship (paddle boat on the Mississippi).
The traditional approach for harnessing biomass chemical exergy is combustion to produce electricity. Biomass combustion produces heat, which produces steam, powers turbines, and creates electricity, which powers our electrified society. A great example of this approach is the UK's biomass combustion facility operated by DRAX. CO2 produced by combustion is released into the air, and while the process is carbon neutral, it is not ideal.
Concerning CDR, there are three strategies for managing biomass chemical exergy;
Mismanage chemical exergy
Utilize chemical exergy
Harness and manage chemical exergy optimally
Direct biomass storage mismanages chemical exergy by transforming biomass into an inert form (solid, oil, etc.) and isolating it from the environment. Although the biomass exergy remains, it is never used to perform work or generate electricity. Additionally, the nutrients in the biomass often cannot be recycled back into the environment.
Processes that utilize biomass exergy include pyrolysis, which synthesizes biochar and converts biomass into valuable fuels like syngas, bio-oil, hydrocarbon fuels, and Sustainable Aviation Fuel. While these processes are generally carbon-neutral, they require additional carbon-free energy to produce high-quality fuels. In these cases, nutrients are often recycled back into the environment.
Another process that utilizes biomass exergy is combustion. Although carbon-neutral, current biomass combustion processes cannot cost-effectively capture the emissions produced, resulting in CO2 being released back into the atmosphere.
Optimal management of biomass exergy involves combusting biomass to generate electricity while cost-effectively capturing and durably sequestering the resulting CO2 emissions. Surplus electricity can then be used to remove 1.5 additional CO2 from the air through Direct Air Capture (DAC). This optimal management is achievable when CO2 capture technology is highly energy-efficient and universal, working with any CO2 concentration. In this scenario, nutrients are recycled back into the environment.
Carbon Credits
Most biomass approaches earn one credit for processing one ton of CO2 -eq. Companies using biomass (1-ton CO2 -eq) to produce fuels and utilize exergy are limited to receiving one credit. Similarly, companies mismanaging exergy with biomass (1-ton CO2 -eq) are limited to one credit for sequestering one ton of CO2 -eq.
The combined biomass combustion and DAC approach will earn 2.5 credits because the biomass's exergy is used optimally, and 2.5 tons of CO2 are durably sequestered per 1-ton CO2 -eq of biomass.
Obviously, companies earning 2.5 credits for biomass will have more leverage in purchasing biomass feedstock than those earning one credit for the same biomass.
Energy Efficient CO2 Capture
Optimal use of biomass for CDR via combustion is impossible today because CO2 capture technologies are energy-intensive, making them cost-ineffective. Today’s CO2 capture technologies are energy intensive because they destroy the feed’s carbon exergy. Just as some biomass CDR approaches mismanage the chemical exergy of biomass, today’s CO2 capture technologies mismanage the carbon exergy of biomass flue gas.
Assessing and quantifying that a CO2 capture technology destroys carbon exergy is easy. Conceptually, evaluate a 950,000-ppm stream being processed through a CO2 capture system. The theoretical minimum energy for processing a 95% pure stream of CO2 into a pure stream of CO2 is very small, 0.003 GJ/ton.
Today’s best CO2 capture systems need at least 2 GJ/ton, and most will need more than 3 GJ/ton. These technologies require more than 2 GJ/ton to accomplish a 0.003 GJ/ton task, which explains why CO2 capture (CCS) has a long history of failure.
A CO2 capture technology that harnesses the feed’s carbon exergy and is capable of Partial Pressure Staging will use ten progressive stages and about 0.1 GJ/ton
Furthermore, because today’s CO2 capture technologies destroy the feed’s carbon exergy, they are not thermodynamically responsive. They can only effectively operate in a single stage. They are incapable of Partial Pressure Staging, where CO2 is removed from the flue gas at higher concentrations, and the energy required is reduced.
I have written several essays discussing the disadvantages of exergy destruction and the benefits of harnessing carbon exergy.
XCaptureCO2 | mike landmeier | Substack
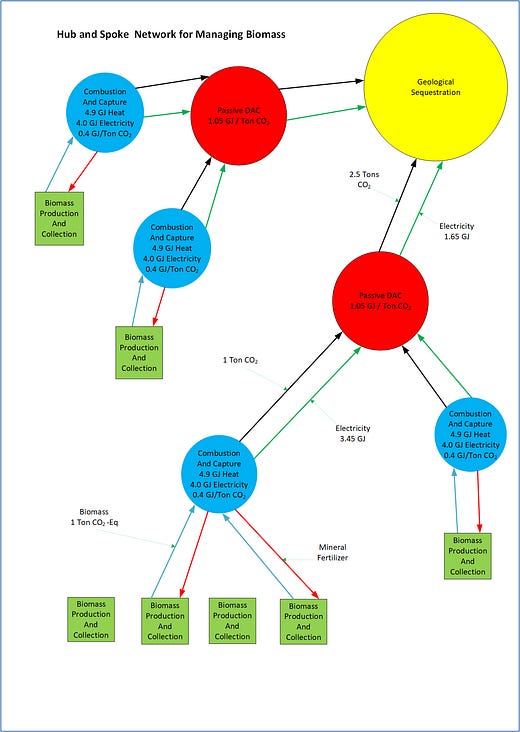
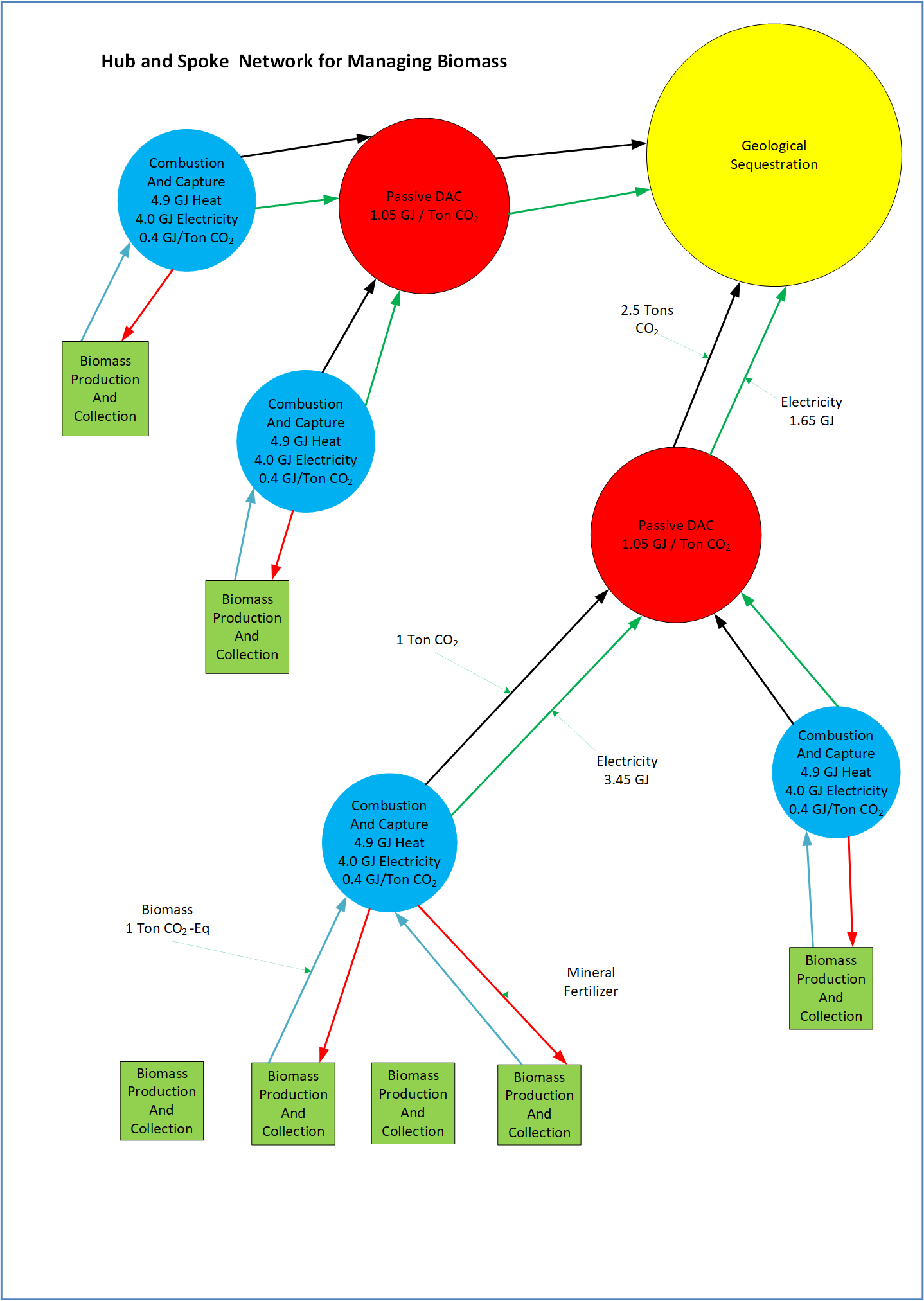
Hub and Spoke Network for Carbon Harvesting
Figure 1. Concept Model
The proposed network involves several specialized facilities – Production, Combustion, DAC, and Sequestration.
Biomass production facilities are farms or facilities that process or collect biomass from the environment. The biomass is minimally processed at this location before being transported to the biomass combustion facility.
The biomass combustion facilities will appropriately prepare the biomass for combustion. This involves drying, necessary pretreatments, grinding, and pelletizing. One optimization is to dry the biomass using the combustion waste heat.
The combustion facility will perform six critical tasks;
Prepare biomass for combustion. This involves drying, necessary pretreatments, grinding, and pelletizing.
Combust the biomass pellets (0.55 tons, 1 ton CO2-eq) to create heat[1] (8.90 GJ) and one ton of CO2.
Use modern techniques such as gasification integrated combined heat and power to produce electricity – achieving 45% efficiency[2].
Produce 4.0 GJ of electricity.
Use 4.9 GJ of waste heat to dry biomass during pelletizing.
Capture CO2 produced by biomass combustion (1 ton of CO2)
Scrub flue gas of undesirable gasses (nitrogen oxides)
Capture CO2 using 8-stage CO2 capture technology (Partial Pressure Staging)
Consume 0.4 GJ to capture produced CO2
Consume 0.15 GJ for mild compression for regional CO2 transport
Process ash produced by biomass combustion into a mineral fertilizer.
Remove heavy metals if necessary
Recycle important nutrients
Transfer products
Transport captured CO2 (1 ton) to the DAC facility
Transport surplus electricity (3.45 GJ) to the DAC facility
Transport the mineral fertilizer back to the preparation facility
The DAC facility uses the surplus electricity produced by biomass combustion to capture and sequester additional CO2.
The DAC facility will receive the 3.45 GJ and remove 1.5 tons of CO2 from the air. Using an energy-efficient CO2 capture technology, 1.05 GJ is used to passively remove CO2 from the air. An additional 0.15 GJ/ton is used to mildly compress the CO2 to transport the DAC CO2 to the CO2 sequestration facility. The total energy to capture and transport 1 ton of CO2 from the air is 1.2 GJ/ton.
The DAC facility will consume 1.5 * 1.2 = 1.8 GJ to capture and transport 1.5 tons of DAC CO2. This leaves a balance of 1.65 GJ, which is transported to the CO2 sequestration facility.
The DAC facility receives one ton of CO2 from the combustion facility and transports all 2.5 tons to the CO2 sequestration facility.
The CO2 sequestration facility is the last facility to process captured CO2.
The CO2 sequestration facility receives 2.5 tons of mildly compressed CO2 and 1.65 GJ of electricity. This CO2 is further compressed to 150 bars using 0.3 GJ/ton. For 2.5 tons, this uses 0.75 GJ. This leaves a balance of 0.9 GJ. This 0.9 GJ of excess electricity is used as needed throughout the process. This surplus electricity will most likely be used for biomass grinding and turning biomass ash into a mineral fertilizer.
Costs
The following is the cost breakdown. The Capex for the biomass combustion and DAC facility is targeted at $62 per ton. Non-energy OPEX is targeted at $30 per ton. Operating profits are targeted at $12 per ton.
The cost of managing biomass ash is targeted to be $7.5 per ton of biomass CO2 -eq, which is $3 per ton captured and sequestered.
These costs total $107 per ton of CO2, including biomass and DAC. This approach allows DAC to be implemented without using other forms of renewable energy, such as solar or wind.
This process meets the target of $120 per ton as long as the cost of energy, which is the cost of biomass delivered, is below $32 (2.5 * $13) per ton CO2-eq. The DOE’s target price of $100 is meaningless without timeframe and inflation guidance. The $120 per ton is OK for 2025 estimates.
Notably, any CO2 emissions attributed to the biomass are distributed across 2.5 tons of captured CO2, not just the source biomass.
With a final biomass combustion CO2 emission concentration of 120 ppm, 1000 kgs of CO2 -eq will release 1 kilogram of CO2 into the air. If biomass acquisition releases 20 kgs of CO2 and preparation/transport releases 14 kgs, then 35 kgs of CO2 are attributed to the 2500 kgs captured. The capture efficiency for this approach is (2500 - 35)/2500 = 2465/2500 = 0.986
This means 1.014 tons (CO2 -eq) of biomass can earn 2.5 credits.
Community Benefits – Energy Supply Balancing for Renewable Energy.
An opportunity for time-of-day energy arbitrage should be evaluated.
The baseline scenario has biomass combustion operating at a constant rate. Biomass is combusted at a steady rate 24 hours a day. The produced emissions are captured and sent to the regional DAC center. The surplus electricity is also sent to the regional DAC hub, which powers DAC and sequestration.
One exciting scenario provides a community benefit of load-balancing renewable energy. Consider a solar-powered community grid producing excess electricity for 8 to 10 hours daily. The excess electricity can power the DAC machines, while biomass combustion operates at a much lower rate. When solar energy declines, the biomass combustion system spins up and powers the DAC system. The biomass combustion rate also increases to create excess electricity sent to the grid to compensate for reduced solar electricity.
The total biomass consumption is the same on a 24-hour basis. However, since biomass combustion varies, biomass combustion to produce electricity compensates for the variable production of solar-powered electricity without the need for batteries.
Universal CO2 Capture Technology
Optimizing Performance by Properly Managing Carbon Exergy
CO2 in the atmosphere is very dilute and expensive to capture. Biomass is produced naturally from the dilute CO2 in the air. However, when combusted, the CO2 in the flue gas emission is very high, ranging from 14-20%. Biomass combustion provides an opportunity to capture CO2 at a much higher concentration, which requires much less energy, according to the thermodynamics suggested by the Thermodynamics of mixing gases.[3]
In this way, technology can work with natural processes to reduce energy consumption and remove CO2 from the atmosphere.
The key to this strategy is energy-efficient CO2 capture. The DOE’s general focus on CO2 capture is misguided. It focuses not on fundamental energy efficiency but on efficiency based on the use of waste heat. The lack of success for effective CO2 capture from flue gas is a significant clue that the strategy is not working. CO2 capture projects that attempt to capture CO2 from coal, biomass, and natural gas flue gas are routinely canceled because they are not cost-effective. They use too much energy. Also, these implementations are very complex. The mean time between failures is very low, causing poor performance.
What will work is a CO2 capture chemistry that is fundamentally energy efficient. This CO2 capture chemistry is universal as it works precisely the same regardless of the type of flue gas. Most importantly, this universal CO2 capture chemistry is thermodynamically responsive; it uses less energy as the CO2 concentration in the flue gas increases.
Thermodynamically responsive CO2 capture is possible because this novel chemistry harnesses, not destroys, the feed's carbon exergy. This is helpful, but it is not the most important benefit. Preserving the feed's carbon exergy allows for “Energy-Optimized Gradient Capture” (EOGC).
Most CO2 capture technologies (DAC, CCS) use a very strong capture material capable of capturing CO2 from very dilute sources. These materials can capture CO2 from mixtures containing less than 1 part per million and even 1 part per billion. This strong capture capability destroys the feed's carbon exergy, evidenced by the high energy required to regenerate the capture material. Most CO2 capture technologies capture 90% of the CO2 in the feed in a single pass.
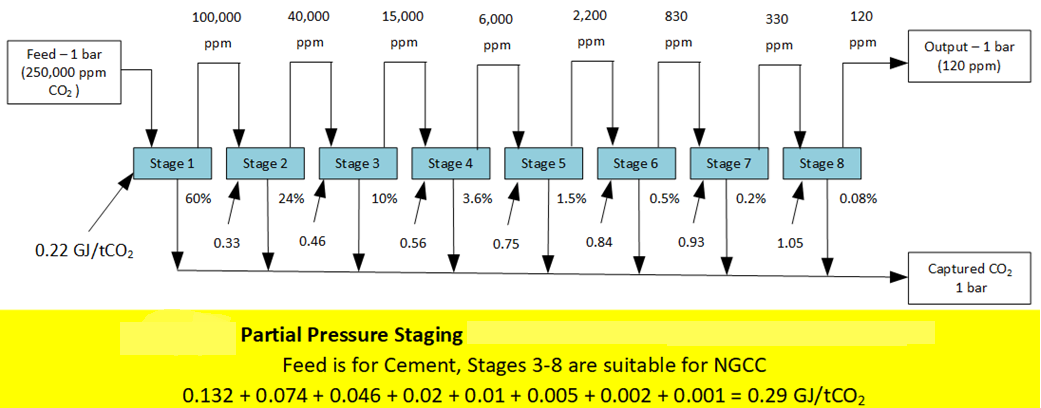
Partial Pressure Staging
A CO2 capture technology that preserves carbon exergy is capable of “Energy-Optimized Gradient Capture (EOGC),” which realizes the benefit of capturing CO2 at higher concentrations. Instead of capturing 90% of CO2 in a single pass, EOGC only captures 60% of the CO2 in a single pass (or stage). The energy to regenerate the solvent is inversely proportional to the CO2 concentration in the feed. So, the first stage captures CO2 at the highest concentration but uses the least amount of energy (per mole).
The output of the first stage is fed to a second stage, which again captures 60%. While this stage uses more energy per mole, this stage will only capture 24% of the CO2 from the original feed. After two stages, 84% of the original feed’s CO2 has been captured at much higher partial pressures.
A third stage will capture 60% of the CO2 from the second stage's output, which contains 16% (100 – 84) of the CO2 from the original feed. Again, the 3rd stage will use more energy per mole, but it only captures 9.6% of the original feed’s CO2. At the end of the 3rd stage, 93.6% of the CO2 from the original feed has been captured.
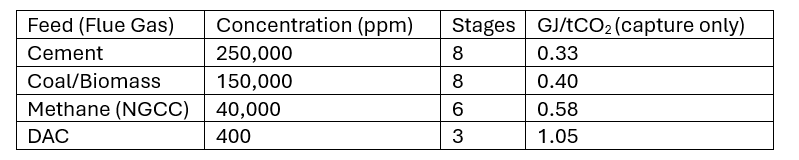
Capturing CO2 at much higher concentrations significantly reduces the energy required. Models using eight stages, with a final output of 120 ppm, show that CO2 can be removed from biomass combustion flue gas (150,000 ppm) using 0.4 GJ/ton.
The CAPEX for the eight stages of CO2 capture may seem overkill, but the benefit of capturing 99.97% of the produced emission provides excellent benefits for the business model.
Figure 2 shows a universal CO2 capture technology that harnesses the feed’s carbon exergy and is capable of Partial Pressure Staging.
Figure 2 – Partial Pressure Staging -- Concentration Gradient CO2 Capture.
Figure 3 – The Effects of Partial Pressure Staging – Capturing more CO2 at higher concentrations requires less energy.
[1] Combustion of Fuels - Carbon Dioxide Emission (engineeringtoolbox.com)