Energy efficient CO2 gas separation requires Partial Pressure Staging, which requires proper exergy management
Thermodynamics tells us the best way to solve challenging problems
Today’s leading CO2 gas separation technologies are awful because they are incapable of partial pressure staging, which is needed to achieve energy-efficient operation. Despite decades of development and billions of dollars spent on R&D, these technologies are limited to single-stage operation. This starkly contrasts with Rocket scientists who solved their staging problem in the 1950s. Continued investment into these horrible chemistries is nothing more than Thermodynamic Idiocy[i].
This essay will explore what appears to be a neglected analysis of CO2 gas separation technologies: CO2 capture from flue gas and from the air. This analysis closely relates to developing a conceptual nature-inspired CO2 gas separation technology.
But first, some people, like the Godfather, prefer to hear bad news up front. So, here it is. Everyone to whom I have introduced it over the past 18 months has rejected the concept driving this essay. This includes NSF (twice), Breakthrough Energy Ventures (twice), Milkywire (twice), DOE (three times), FrontierClimate (once), and a few others. These funding audiences continue to support CO2 gas separation processes that maintain the status quo and disrespect both common sense and the laws of thermodynamics. They prefer this concept to remain science fiction permanently.
Nothing presented here is peer-reviewed. However, the technology underlying this essay was developed during my nine-year investigation of the molecular biology of living organisms and their ability to acquire and manage environmental CO2. This effort allowed me to develop an understanding of how the strategies, chemistries, and technologies of living organisms manipulate CO2 with little energy. I also studied most other CO2 capture technologies and am generally knowledgeable about our technological civilization.
My study of living organisms focused on ocean-dwelling organisms such as kelp, diatoms, and Cyanobacteria (blue-green algae). After 3.8 billion years of evolution, living organisms properly manage exergy while acquiring CO2 from the environment. Living in a light-attenuated underwater environment, Cyanobacteria preserve and harness feed exergy, which is crucial for their survival and the propagation of their species. I have successfully modeled a CO2 gas separation technology based on the biochemistry of living organisms, such as Cyanobacteria.
This nature-inspired CO2 gas separation technology was validated by the success of the Moonshot program. The DOE is using the Moonshot's success to promote its work to decarbonize our fossil fuel-dependent civilization. The amazingly successful Moonshot required many innovations, chief of which was the development of the multi-stage Saturn V rocket and the Apollo spacecraft. The Saturn V is a visible demonstration of success, and that success is because of the rocket’s ability to properly manage exergy and implement staging.
With this experience, I can now ask one unexpected question: What do Cyanobacteria and the Saturn V rocket have in common? I assert they both fully respect the laws of thermodynamics and properly manage exergy.
I have four claims to present;
1. Current CO2 gas separation technologies are awful because they destroy the carbon exergy of the feed.
2. The destruction of exergy during the gas separation process prevents staging.
3. Staging is critical to significantly reducing the energy required to remove CO2 from gas mixtures. Successful staging will dramatically reduce the energy necessary for CO2 separation from cement, coal, biomass, and methane flue gases. It will also dramatically reduce the energy necessary for arbitrarily scalable, gigaton-plus CO2 Direct Air Capture.
4. CO2 gas separation will not be successful until the capture technologies are more like Cyanobacteria and the Saturn V rocket. They must respect thermodynamics and properly manage exergy.
So, what is exergy?
I asked Microsoft Copilot (ai) this question and received this answer. The answer is very long and detailed, but here is the first paragraph.
“Exergy, often referred to as “available energy” or “useful work potential,” is a fundamental concept in the field of thermodynamics and engineering. It is crucial for understanding and quantifying the quality of energy within a system and its potential to perform useful work. Exergy represents the maximum work that can be obtained from a system as it reaches equilibrium with its environment through an ideal process.”
This answer is quite common and often involves analyzing how much work can be performed from heat energy. While this answer is long, I suspect it is also abbreviated. The total answer included elements that I disagree with.
The following definition of exergy is the centerpiece of my favorite exergy-centric document.
Lecture 5 Exergy.pdf (mit.edu)
Figure 1 (from Page 24).
Figure 2 (from Page 25).
The authoring professor presents the exergy definition in an unusual way. The slide on page 24 shows a simple definition of exergy that aligns well with the AI definition of exergy. Interestingly, the professor uses the next page to expand the definition of exergy. I don’t know the professor’s reasoning for presenting the definition this way, but it appears that the first definition is commonly known while the 2nd definition is somewhat ignored.
The second part of this definition is critical to my work and this analysis. However, understanding these definitions in their entirety is a bit challenging. I spent countless hours working to understand the concept, and then, after a task switch, I found I needed to start over to learn it again. Doing this a few times caused me to develop a helpful mnemonic.
So, here is Mike’s Working Definition of Exergy
1. Exergy is that which you have that helps you achieve a goal.
2. Exergy is situational relative to a goal.
3. What you have are the components of the reference, and the system is the goal.
To complete the definition of exergy, I include the following information. The exergy of a system is the sum of various kinds of exergy, including physical, chemical, kinetic, and potential, given as
Ex = EPH + ECH + EKN + EPT EQ1
Exergy is the maximum useful work that can be extracted from a system. For instance, a $100 bill has exergy. In this case, the reference is the $100 bill, and a goal like purchasing a $1000 TV set is the system. The “useful work” it can do is its purchasing power - the goods or services it can buy. Like exergy, the $100 bill can be used efficiently or wasted. If you use it wisely, you can get goods or services that are valuable to you. This is like a system using its exergy efficiently to do useful work. However, if you waste the $100 bill, say by burning it, you destroy all its exergy. The opportunity to use it for something useful is lost, just like when energy is wasted in a system; its exergy decreases.
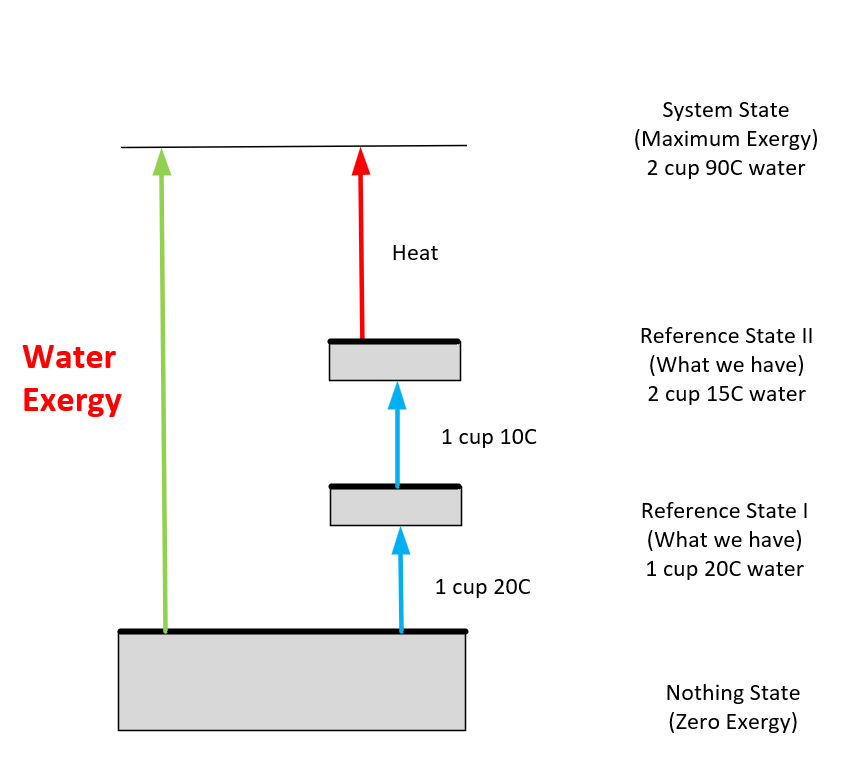
Also, how you use this $100 bill can affect how much ‘work’ you extract from it. For example, if you’re feeling cold, you could burn the $100 bill for heat, but it would only provide a small amount of heat for a short time. If, instead, you used the $100 to buy a blanket or a heater, you could stay warm for a much longer time. This is an example of managing exergy efficiently. So, the $100 bill is a good metaphor for understanding exergy - it represents potential that can be used efficiently, wasted, or mismanaged. I use a three-state analysis (Figure 3) to visualize this mismanagement and destruction of exergy, which has an additional nothing state rather than the traditional two-state exergy description.
Figure 3 – Helpful exergy analysis mnemonic.
The nothing state has nothing or extremely little that helps achieve the goal. These three states allow us to easily quantify the exergy we have.
Discussing exergy is always challenging. Directly discussing exergy management of CO2 gas separation technologies is beyond challenging. I need to build a less technical foundation to properly explain exergy management using today's CO2 capture technologies. So, I have created a simple demonstration to help everyone understand the issues.
For the following exergy analysis, it is essential to understand that CO2 in a gas mixture or water in a cup is chemical exergy (referred to in EQ1). This is generally not obvious because neither CO2 nor water have heat exergy (like a fuel). Nevertheless, they are both capable of doing work under the right circumstances. Depending on the goal, they are both things we have relative to our goal.
The following exergy discussion is simplistic, silly, and intentionally antagonistic. It is also very useful.
Is a cup of 20C water exergy? Once again, it depends on the goal. If we want one cup of sugar, the cup of water has no exergy.
However, if our goal is to have 2 cups of 90C water, then our cup of 20C water is helpful. We have half the required mass and a good amount of heat.
Our friend, the rocket scientist, demonstrates one possible strategy to accomplish our goal.
The rocket scientist has a cup of 20C water and wants two cups of 90C water. He consults his rocket scientist manual, which states that step 1 is to conserve exergy. The rocket scientist uses a second cup and fills it with 10C water from a faucet. He again consults his rocket scientist manual, which states that the next step is to manage entropy carefully. He puts both cups of water into a pan and puts it on the stove. He adds heat until the water has a temperature of 90C. Goal accomplished! The following analysis diagram (Figure 4) summarizes the above process.
Figure 4 – Exergy changes to accomplish the goal.
Figure 5 - “Magic Teacup Ride” at Disneyland.
This example is simple enough. A simple plot (Figure 5) shows that exergy and entropy gently transition from the starting point to the endpoint. In roller coaster terms, this is the “Magic Teacup Ride” at Disneyland, a favorite for all 5-year-old children.
Thermodynamics forces us to realize that there are real limits to how well a process may perform. However, thermodynamics does not limit how poorly a process may perform.
Our friend, the chemical engineer, will demonstrate such a bad process.
The chemical engineer has a cup of 20C water and wants two cups of 90C water. He consults his chemical engineer manual, which states that step 1 is to destroy exergy. The chemical engineer dumps the water onto the floor, creating a mess and contaminating the water. He uses energy to clean up the mess and then uses two cups to get two cups of 10C water from the faucet. He consults his chemical engineer manual again, which states that step 2 is to mismanage entropy.
He first uses energy to reduce the temperature of the two cups of water from 10C to 0C. He then uses energy to turn the 0C water into 0C ice (a solid). He then uses energy to heat the 0C ice into 0C water.
Are we back on track? Of course not.
He then uses energy to warm 0C water to 100C, overshooting the temperature goal. Then, he uses energy to turn 100C water into 100C steam (gas). He then uses energy to chill the 100C steam into 100C water.
Finally, he uses energy to chill the 100C water to 90C—a mission accomplished in true chemical engineer fashion.
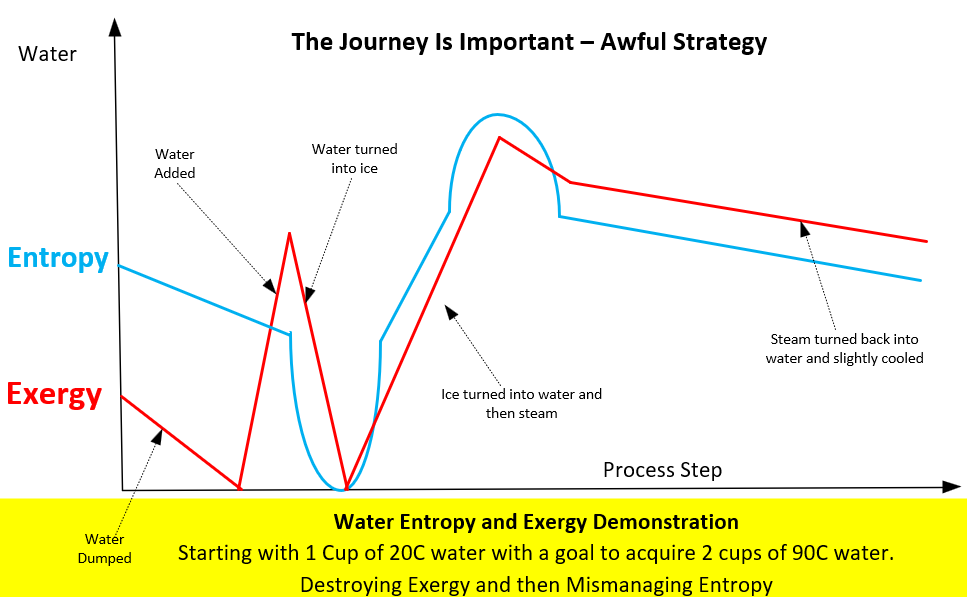
Figure 6 – Thermodynamics places no limits on bad processes.
A simple plot (Figure 6) shows that exergy and entropy both experienced a wild ride from the starting point to the endpoint. In roller coaster terms, this is the Valravn Dive Coaster, shown in Figure 7.
Figure 7 - Valravn Dive Coaster at Cedar Point.
This rollercoaster ride is terrifying, even for rollercoaster enthusiasts. Five-year-old children are generally crying before the ride even begins.
*** Interestingly, rollercoasters like this manage exergy fairly well, as they routinely transform electrical energy into potential exergy and then into kinetic exergy and often back to potential exergy. Exergy is destroyed by braking, which turns kinetic energy into heat. ***
As demonstrated here, the destruction of exergy and mismanagement of entropy is absurd. Unfortunately, the destruction of exergy and mismanagement of entropy shown in this example using cups of water highly correlate to many of today’s leading CO2 gas separation technologies. CO2 flue gas capture projects are routinely canceled after billions of dollars are spent, with chemical engineering managers declaring, ”The technology works! It’s just not cost-effective.” This example illustrates why today’s CO2 gas separation technologies are not cost-effective.
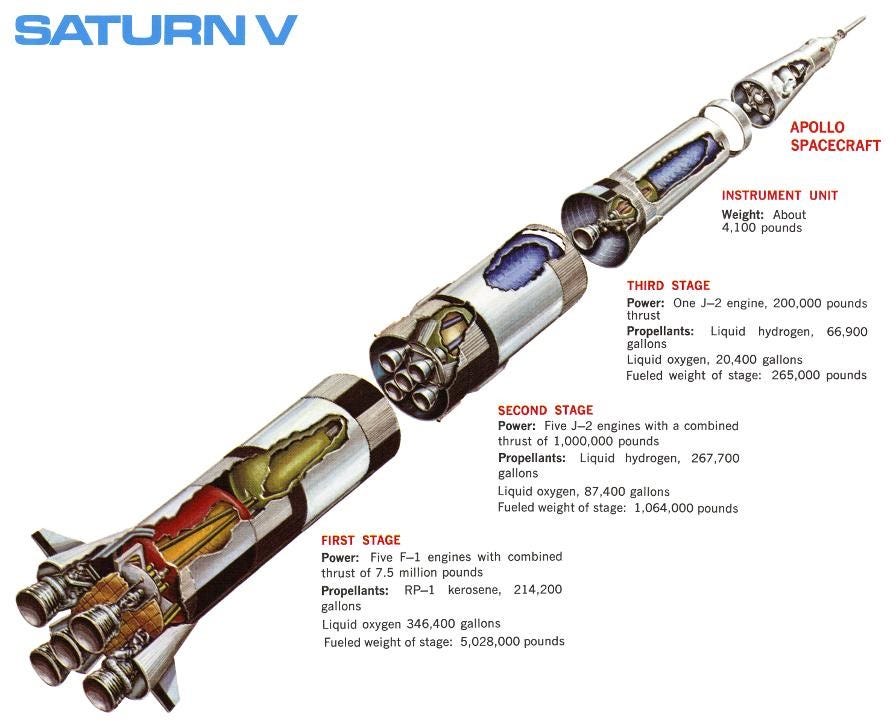
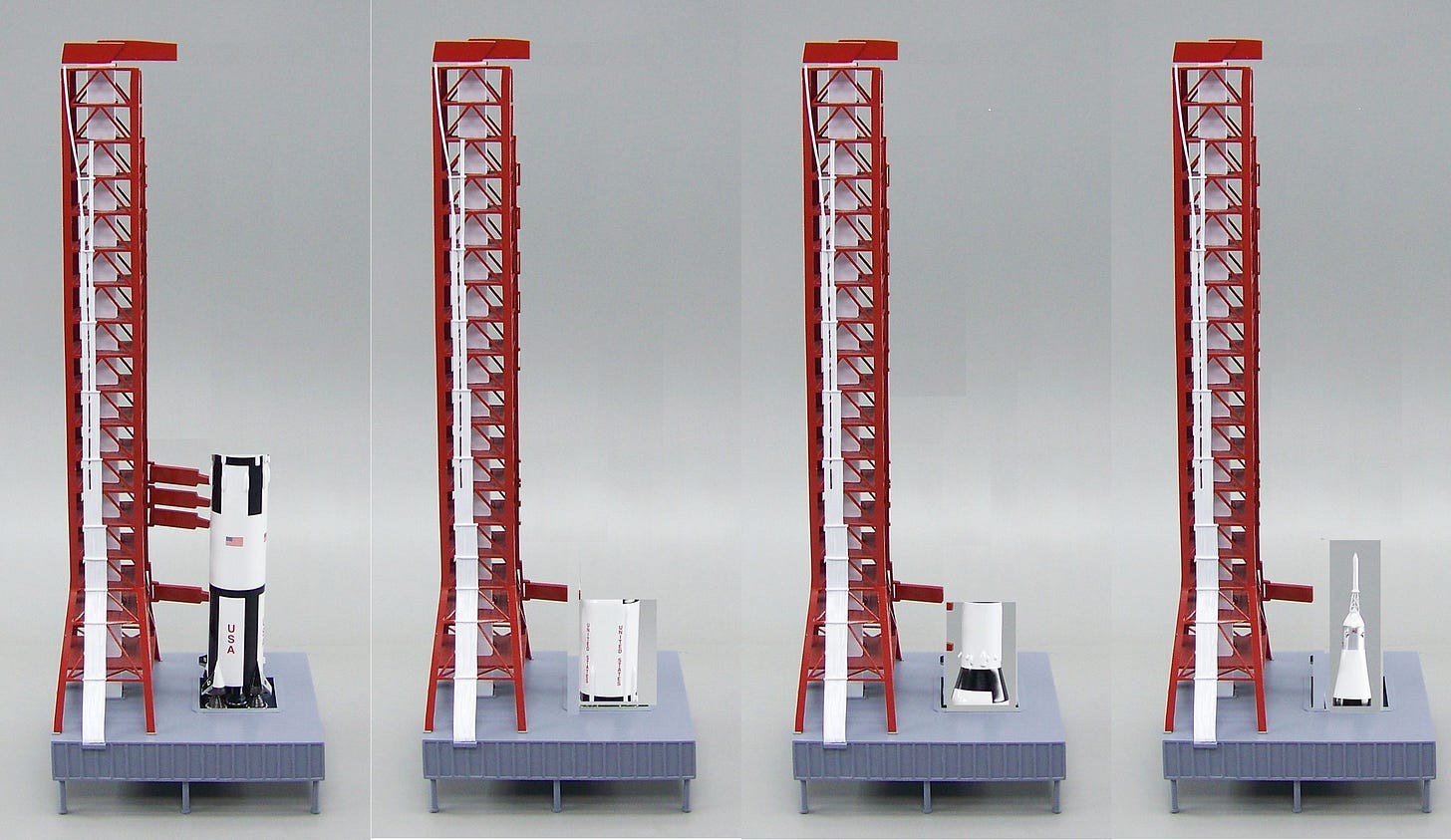
Properly managing exergy is critical to accomplishing energy-intensive tasks. Rocket scientists provide one of the best examples of exergy management, as demonstrated in Figure 8.
Figure 8 – One of the greatest managers of exergy. First place belongs to Cyanobacteria.
The Saturn V/ Apollo spacecraft is among the best examples demonstrating the successful management of exergy. At the instant of first-stage ignition, all the exergy (useable system work) necessary to complete the mission is contained within the rocket. This includes the exergy required to send a large mass (47 tons, containing three men) into low earth orbit, leave earth orbit, travel to the moon, enter moon orbit, separate the LEM, deliver two men to the surface of the moon, have a crew stage leave the moon’s surface and rejoin the command module, leave the moon’s orbit, travel back to earth, renter the earth’s atmosphere and then land safely back on the earth’s surface.
The entire mission, from start to finish, is completed without refueling. Exergy is a critical resource, so properly managing exergy is a primary design consideration for every aspect of the mission.
Figure 9 – Vertical Rocket
A vertical rocket on a launch pad is demonstrated in the following image (Figure 9). At lift-off, the bulk of the rocket’s exergy is chemical exergy contained in the fuel. The earth’s rotation causes a small amount of kinetic exergy.
Once the first stage is ignited, chemical exergy is converted into thermal exergy, which is then converted into kinetic exergy. Finally, some of the kinetic exergy is converted into potential exergy. During the launch, the bulk of the chemical exergy is used to overcome various losses, including Aerodynamic Drag, Engine Efficiency, Incomplete Combustion, Fuel Gravity Drag, Jettisoned Stages, and Radiation Losses. These losses are unavoidable in achieving the goal of getting the payload into orbit.
However, some chemical exergy is converted into kinetic and potential exergy in the payload. Each stage operates sequentially and transfers some chemical exergy to the payload, propelling the payload into orbit.
For the moon mission, the orbiting payload’s kinetic and potential exergy, supplemented by additional chemical exergy, is used to travel to the moon and back.
The critical point here is that transferring exergy during a complicated, energy-intensive process eventually provides options to use that exergy to do useful work (achieve goals). Destroying exergy denies the opportunity to use that exergy and accomplish a goal.
Chemical engineers would design a rocket very differently. They would prioritize exergy destruction using principles adopted from CO2 gas separation technologies. Chemical engineers would develop the horizontal rocket, as shown in Figure 10.
Figure 10 – Horizontal Rocket
The horizontal rocket destroys exergy by
1. Turning chemical exergy (fuel) into heat.
2. Not doing work on the subsequent stages and payload.
3. Failing to transfer the rocket’s chemical exergy into kinetic and potential exergy of the payload.
Once again, the horizontal rocket is a silly concept that obviously does not and will never work. Unfortunately, today’s CO2 gas separation technologies have much in common with this horizontal rocket.
While the Saturn V consumes a lot of feed exergy, that's the job. Despite the significant exergy losses, it successfully transfers enough exergy to the Apollo spacecraft to complete the moon mission. In contrast, today's CO2 gas separation technologies destroy feed exergy, usually by step 3.
My study of CO2 management chemistries has convinced me that properly managing exergy is the key to managing CO2. Living organisms do not use 900C calciners or waste heat. Their chemistry is fundamentally energy efficient.
This narrative leads to the point of this essay. The destruction of exergy prevents successful staging. Without staging, massive payloads are not delivered into low-earth orbit. Without staging, the CO2 capture performance shown in Figures 11 and 12 is impossible.
Figure 11 – Partial Pressure Staging -- Permanent Science Fiction?
Figure 12 – Cost-effective, profitable CDR credits -- Not going to happen.
The exergy demonstration using water is neither arbitrary nor random. It was carefully constructed to correlate highly with today’s best CO2 gas separation technologies. That analysis shows why today’s CO2 DAC technologies need more than 4 GJ/tCO2 to complete 0.43 GJ/tCO2 tasks. Exergy destruction of about 1.1 GJ/tCO2 is a significant reason the required energy is so great. Exergy destruction during flue gas capture is even worse because the feeds have more exergy. Performance this poor is not accidental and does not get fixed.
Continued public funding of these technologies is a social welfare program for engineers and scientists seeking to get paid. Public funding of these poor technologies pays smart people to do stupid things.
We should develop a CO2 gas separation technology that manages CO2 in a way comparable to Cyanobacteria. What's the worst that can happen? If CO2 gas separation technologies managed exergy as carefully as Cyanobacteria and the Saturn V rocket, policymakers would have a tremendous variety of options to solve the excess CO2 problem. Furthermore, a dramatically cheaper CO2 capture technology will greatly benefit the emerging e-fuel and carbon utilization industries.
Someday, someone (#SpaceX, #NASA, DOD) will actually need an energy-efficient CO2 gas separation technology. That will be a good time for the developing engineers to begin respecting common sense and the laws of thermodynamics. Just say no to continued Thermodynamic Idiocy.
“This is the CO2 capture technology you’re looking for!”, apologies to Star Wars.
When the technology underlying this essay is developed by whomever, most existing CO2 capture / CO2 gas separation approaches become obsolete.
Technical pitch deck
https://drive.google.com/file/d/1uc7y7S_QLVt2YlZL8QUWnEoSp7zLyMY2/view?usp=sharing
Acknowledgments
The author thanks Nikita Chaudhary for helpful comments on a draft of this essay.
[i] Why Direct Air Capture Sucks (and not in a good way!) | LinkedIn












I have been multi-staging water/gas treatment systems for a long time. Nothing new. Getting the first factor of 10 change in concentration is the rough one, after that you have only 10% of the flow rates to deal with.