Carbon Exergy Analysis of Carbon Engineering’s CO2 Capture Process
Understanding why we cannot have nice things.
A Tale of Three Gas Mixtures
Despite being developed for nearly 100 years, today’s CO2 capture technologies are incapable of Partial Pressure Staging. This assertion was explored in my recent essay - https://circularcarboninnovations.substack.com/p/energy-efficient-co2-gas-separation.
Partial Pressure Staging is not possible with today’s CO2 capture technologies because they destroy the carbon exergy of the feed.
This essay will quantitatively analyze carbon exergy destruction during CO2 capture using the best CO2 capture technologies available today. While carbon exergy destruction is common to most CO2 gas capture technologies, this essay will specifically focus on Carbon Engineering’s CO2 capture process, as documented in this paper: A Process for Capturing CO2 from the Atmosphere - ScienceDirect. While this paper provides a lot of good information, Figure 2 is outstanding for this analysis.
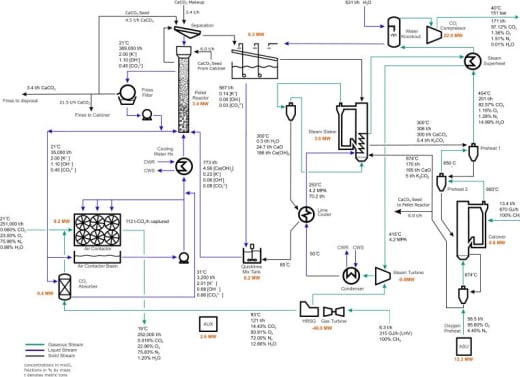
Figure 2. Overview of Process Showing Mass and Energy Balances
Figure 2 contains a lot of activity. I focus this analysis on a few critical sections. I first break the diagram into two sections: the front end and the back end. The front end begins with the fan icon on the lower left and includes the pellet reactor. The back end is everything beyond (to the right of) the pellet reactor to the compressor on the upper right, where CO2 is compressed for sequestration.
My essay discussing exergy destruction states that most CO2 capture chemistries destroy carbon exergy generally within the first three steps. Carbon Engineering destroys the carbon exergy of the feed in the front end as CO2 journeys from the fans to the pellet reactor.
The front end's overall function is to capture CO2 from a gas mixture (air) and transform it into CaCO3 pellets. This process turns components of a gas mixture into a solid and CO2 into something other than CO2. The reason for this is the pellets (a solid) can be easily separated for further processing.
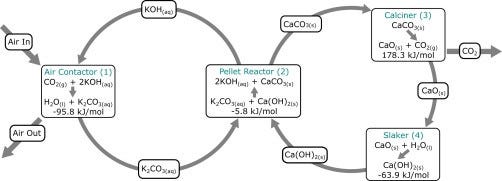
Figure 1 provides a diagram for the overall chemistry. In Figure 1, the front end is the left loop, while the back end is the right loop.
Figure 1. Process Chemistry and Thermodynamics
The CO2-containing gas mixture is blown across an aqueous alkaline solvent inside a carefully designed air contactor. This solvent is a very powerful base solution, generally a 2-molar KOH solution[i]. The solvent’s pH is very high, close to 13.9. EQ1 shows the first reaction of the capture process.
CO2 + 2K+ + 2OH- ==> 2K+ + CO32- + H2O EQ1
The gas containing CO2 enters on the left of the air contactor (fan icon). The blue line from the fan icon shows a liquid stream containing K+ and CO32- ions.
The next step happens at the bottom of the pellet reactor. The CO2-rich solvent from the air contactor is mixed with Ca(OH)2, which causes the following reaction;
Ca(OH)2 ==> Ca2+ + 2OH- EQ2
2K+ + CO32- + H2O + Ca2+ + 2OH- ==> 2K+ + 2OH- + CaCO3 + H2O EQ3
The net reaction from CO2 to CaCO3 is
CO2 + 2K+ + 2OH- + Ca(OH)2 ==> 2K+ + 2OH- + CaCO3 + H2O EQ4
The entire reaction is exothermic, spontaneous, produces heat, and increases entropy.
Once formed, CaCO3 is microscopic. To facilitate its separation from the solvent, a crystallization process using CaCO3 seeds creates CaCO3 pellets large enough for physical separation. Once separated, the pellets are sent to the back end to be processed into a gas using the 900-degree C Calciner, which is on the middle right of Figure 2.
The front-end reaction shown in EQ4 is not reversible at standard temperature and pressure. If the solvent’s circulation was halted, an equilibrium would occur where the net mass transfer is zero. This is a static equilibrium, as the mass transfer is zero for both the forward and reverse reactions.
It is important to note that these reactions are insensitive to the concentration of CO2 in the incoming air stream. Once a CO2 molecule makes contact with the solvent, the reaction proceeds to completion.
This chemistry used by Carbon Engineering can be described as turning the CO2 in a gas mixture into rocks. Since the reaction does not achieve a dynamic equilibrium, the reaction destroys the carbon exergy of the feed.
Carbon Engineering documents its energy use as 8.6 GJ/ tCO2. About 0.45 GJ / tCO2 is used for compression, and about 1 GJ / tCO2 is used for the fans. This leaves about 7.1 GJ / tCO2 for the actual CO2 capture chemistry.
With this data about their chemistry, analyzing carbon exergy destruction using three feed gases is straightforward.
Gas 1 is a synthetic gas comparable to air. It is a nitrogen gas with 400 ppm of CO2. The minimum energy to transform Gas 1 into a pure CO2 stream is determined using the Gibbs free energy:
Emin = RT ln (Cf/Ci). EQ5
With R = 8.314, T = 293, Cf = 1,000,000, and Ci = 400,
Emin is 8.314* 293* 7.82 = 19 Kj/mol EQ6
I prefer to work with GJ / tCO2, so I multiply by 22722 (mol CO2 / ton CO2).
Emin = 22727 * 19KJ = 0.43 GJ/ tCO2 EQ7
This aligns well with Keith’s calculations shown on page 110[ii].
Gas 2 is a synthetic gas representing a very dilute CO2 mixture. It is nitrogen with 1 ppt (part per trillion) of CO2. The minimum energy to transform this gas into a pure CO2 stream is
Emin = 22727 * RT ln (10 ^ 12) = 1.53 GJ/tCO2 EQ8
Gas 3 is a synthetic gas representing a very pure stream. It is pure CO2, having one million parts per million of CO2. The minimum energy to transform this gas into a pure CO2 stream is
Emin = 22727 * RT ln (1,000,000/1,000,000) = 0 GJ/ tCO2 EQ9
This minimum energy for Gas 3 is intuitive as it takes no energy to transform a pure CO2 gas stream into a pure CO2 gas stream.
For this mental exercise, we will process these three gas streams using Carbon Engineering’s front end to transform a gas mixture containing CO2 into CaCO3 pellets. The differing concentrations of CO2 in the feed streams will affect the time it takes to create the pellets, but the thermodynamics of this chemistry are unaffected by time.
For Gas 1, CO2 will flow from the gas feed into the solvent and be turned into pellets, as described before. These pellets will be identified as Gas 1 pellets.
For Gas 2, CO2 will flow from the gas feed into the solvent and be turned into pellets, as described before. These pellets will be identified as Gas 2 pellets.
Lastly, for Gas 3, CO2 will flow from the gas feed into the solvent and be turned into pellets, as described before. These pellets will be identified as Gas 3 pellets.
Regardless of the feed's CO2 concentration, the energy required to process a ton of CO2 is 7.1 GJ / tCO2. Carbon exergy destruction can be documented here.
If we take all 3 kinds of pellets and put them into a single bucket, could we separate them back to their original organization? The answer is no. The pellets are all identical as they are all identical CaCO3 rocks.
We cannot tell the difference between the pellets, nor can the laws of thermodynamics. Thermodynamics does not know or care about the history of CO2. The pellets that exist have no connection to their past. The energy for turning the CaCO3 back into CO2 is the same regardless of the original concentration of the feed. Whatever carbon exergy once existed, that exergy is lost as the CaCO3 pellets are formed.
The documented carbon exergy destroyed is relative to the minimum CO2 feed (Gas 2) used in this example - 1 part per trillion (ppt). For instance, Gas 3 has 1.53 GJ / tCO2 of carbon exergy relative to a gas containing 1 ppt CO2 (Gas 2).
For Gas 1, what was originally a 0.43 GJ / tCO2 task is accomplished using 7.1 GJ / tCO2 of energy. Exergy destroyed is (1.53 – 0.43 = 1.1 GJ / tCO2).
For Gas 2, what was originally a 1.53 GJ / tCO2 task is accomplished using 7.1 GJ/ tCO2 of energy. Exergy destroyed is (1.53 – 1.53 = 0 GJ / tCO2).
For Gas 3, what was originally a 0 GJ / tCO2 task is accomplished using 7.1 GJ / tCO2 of energy. Exergy destroyed is (1.53 – 0 = 1.53 GJ / tCO2).
Once carbon exergy is destroyed, it must be reacquired to achieve our goal of creating pure CO2 gas output. The fact that 7.1 GJ / tCO2 is needed to transform a pure stream of CO2 into a pure stream of CO2 correlates well with carbon exergy destruction and easily identifies a fundamentally bad CO2 capture process.
This carbon exergy destruction is apparent in Carbon Engineering’s CO2 capture process, but it is nearly identical for all Calcium looping, amines, and adsorption CO2 capture processes.
Here is the carbon exergy destruction, relative to Gas 2, for common CO2-containing flue gases -
For Cement (250,000 ppm), what was originally a 0.08 GJ / tCO2 task is accomplished using 7.1 GJ / tCO2 of energy. Exergy destroyed is (1.53 – 0.08 = 1.45 GJ / tCO2).
For Coal combustion (150,000 ppm), what was originally a 0.11 GJ / tCO2 task is accomplished using 7.1 GJ / tCO2 of energy. Exergy destroyed is (1.53 – 0.11 = 1.42 GJ / tCO2).
For Biomass Combustion (150,000 ppm), what was originally a 0.11 GJ / tCO2 task is accomplished using 7.1 GJ / tCO2 of energy. Exergy destroyed is (1.53 – 0.11 = 1.42 GJ / tCO2).
For Methane (natural gas) Combustion (40,000 ppm), what was originally a 0.18 GJ / tCO2 task is accomplished using 7.1 GJ / tCO2 of energy. Exergy destroyed is (1.53 – 0.18 = 1.35 GJ / tCO2).
Today’s CO2 capture processes use materials that bind to CO2 very strongly. They are capable of capturing CO2 from extremely dilute feeds. The minimum energy needed to capture CO2 is determined by the process’s ability to capture CO2 from extremely dilute feeds.
The consequence of carbon exergy destruction by these CO2 capture processes is the inability to cost-effectively remove CO2 from the flue gas of fossil fuel combustion and cement production.
Furthermore, the destruction of carbon exergy prevents Partial Pressure Staging. This concept is possible only if carbon exergy is properly managed. Partial Pressure Staging is impossible if carbon exergy is destroyed.
CO2 capture processes that properly manage carbon exergy could have been developed 20 or 30 years ago. They were not. Currently, most CO2-containing flue gas is vented directly into the atmosphere. Instead of having cost-effective CO2 scrubbing, our civilization releases more than 40 gigatons of CO2 into the atmosphere annually. Due to continued fossil fuel usage and poor CO2 capture technologies, we now have a warming planet.
Looking forward, continued disinterest in energy-efficient CO2 capture technologies that properly manage carbon exergy will mean a tremendous amount of CO2 (many hundreds of gigatons) will be added to the air over the next decades. The heat-retaining properties of CO2 and other greenhouse gases suggest that a tremendous amount of planetary warming will occur in the future.
Carbon exergy destruction and the inability to implement Partial Pressure Staging are why we cannot have nice things, like a cooler climate and a more livable planet.
[i] 148.holmes.keith_.contactorforlargescalecapture.e.pdf (harvard.edu)